
Study Status, Eligibility, Study Design and Study Identification
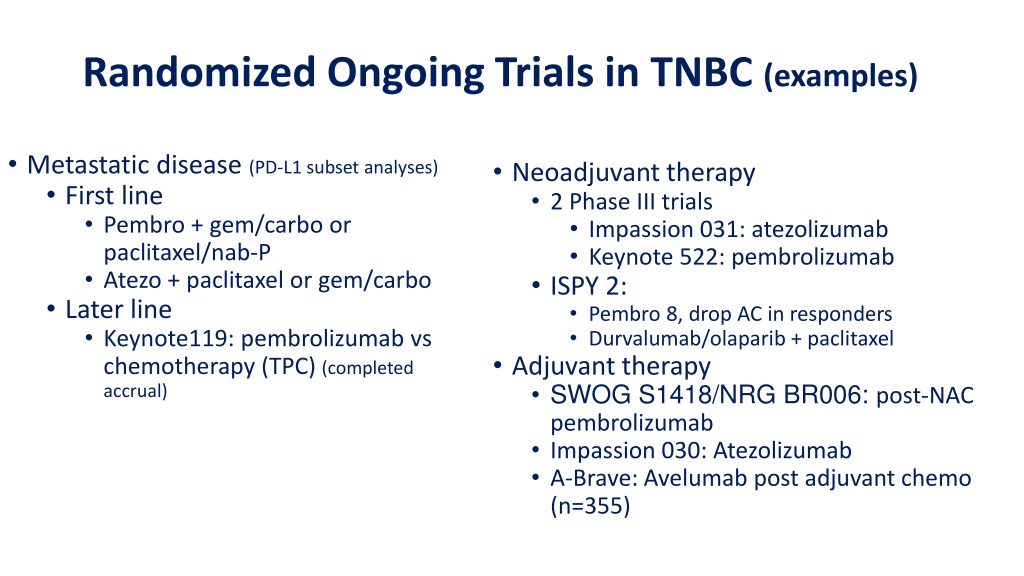
Study Status, References and Contacts/LocationsĪrms and Interventions, Study Design, Outcome Measures, Conditions, Study Status and Eligibility Study Status, Arms and Interventions, References, Contacts/Locations, Eligibility, Outcome Measures, Study Design, Conditions, Study Description and Study Identification Study Status, IPDSharing and Contacts/Locations Study Status, Oversight, Contacts/Locations, Eligibility, Outcome Measures and Study DesignĬontacts/Locations, Study Status and Eligibility Several hundreds or thousands of patients.Recruitment Status, Study Status and Contacts/LocationsĬontacts/Locations, Study Status and Study IdentificationĬontacts/Locations, Arms and Interventions, Study Status, Eligibility and Outcome Measures It studies the side effects caused over time byĪ new treatment after it has been approved and is on the market.

Phase III trials enroll 100 or more patients. Of each drug and which drug works better. The study drug is testedĪmong patients with a specific cancer type and new combinations of drugs may be used.Ĭompare a new drug to the standard-of-care drug. Low doses are given initially to a few patients, higherĭoses are given in others until desired effect is reached or undesirable effects seen.įurther assess safety of a drug in a large sample of patients. The drug is tested inĪ small group of 15 to 30 patients. TheĪim is to find the best dose of a new drug with the fewest side effects. Tests the safety of a drug in healthy volunteers or subjects with indications.

Processed in the body and how it affects the body. The first clinical trials done among people.
#Keynote 158 clinical trials.gov trial#
Steps followed in clinical trial research to obtain sufficient evidence that a process The length of time required to continue contraception for each study intervention is as follows: MK-3475 (120 days) and agrees not to donate eggs (ova, oocytes) to others or freeze/store for her own use for the purpose of reproduction during this period.
(CRC participants will have a histologically proven locally advanced unresectable or metastatic CRC which is dMMR/MSI-H that has received 2 prior lines of therapy) OR Any advanced solid tumor (including Colorectal Carcinoma ) which is Mismatch Repair Deficient (dMMR)/MSI-H in participants from mainland China who are of Chinese descent.Any advanced solid tumor, with the exception of colorectal carcinoma (CRC), which is Microsatellite Instability (MSI)-High (MSI-H) OR.Salivary Gland Carcinoma (sarcomas and mesenchymal tumors are excluded).Endometrial Carcinoma (sarcomas and mesenchymal tumors are excluded).Neuroendocrine Tumors (well- and moderately-differentiated) of the lung, appendix, small intestine, colon, rectum, or pancreas.Biliary Adenocarcinoma (gallbladder or biliary tree (intrahepatic or extrahepatic cholangiocarcinoma) except Ampulla of Vater cancers).Histologically or cytologically-documented, advanced solid tumor of one of the following types:.


 0 kommentar(er)
0 kommentar(er)
